Resources and Support: Clinical Resources
Resources and Support: Clinical Resources
Patient Profiles
Hypothetical patients
Maya’s background
Just before her 2nd birthday, Maya’s dad noticed that she was short of breath and she seemed to have difficulty swallowing
Around the same time, she had a persistent fever and appeared pale
Noah's background
3 years ago, Noah was diagnosed with INSS stage 4 high-risk neuroblastoma with disease present in his chest and metastatic disease in the bone
FISH testing showed no MYCN amplification
Kai’s background and disease characteristics at diagnosis
A little more than 5 years ago, Kai was diagnosed with INSS stage 4 high-risk neuroblastoma with disease present in his abdomen and lymph nodes
FISH testing showed MYCN amplification

Maya’s background
Just before her 2nd birthday, Maya’s dad noticed that she was short of breath and she seemed to have difficulty swallowing
Around the same time, she had a persistent fever and appeared pale
-
Disease characteristics at diagnosis
INSS stage 4 high-risk neuroblastoma with persistent disease in bone and bone marrow after induction therapy
FISH testing: no MYCN amplification
Soft tissue disease:CT scan showed primary tumor in the abdomen with additional metastatic soft tissue lesions in the abdomen and chest
Bone and bone marrow disease MIBG scan and bone marrow aspiration revealed involvement of both bone and bone marrow
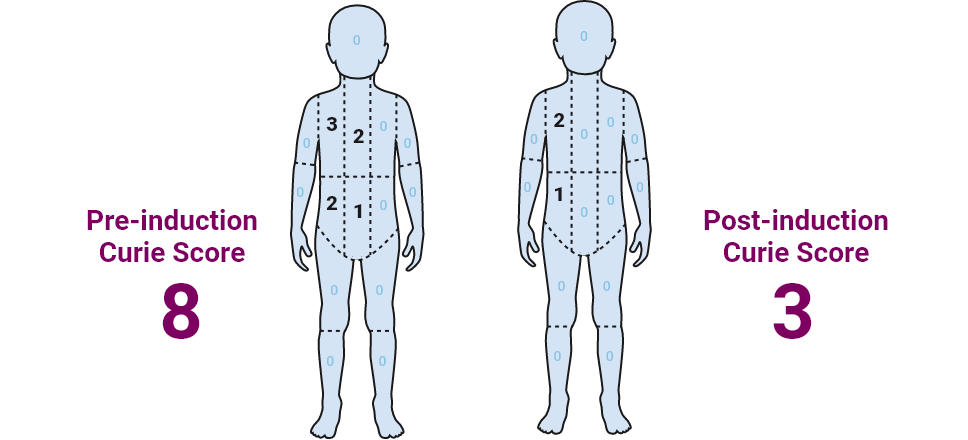
Curie score: 8
Induction therapy
Included 5 cycles of chemotherapy and surgical resection of the primary tumor
Status after induction therapy: partial response
Metastatic soft tissue disease: resolved
Bone and bone marrow disease: resolved
Curie score: 3
Considerations for next steps
Maya has residual disease in the bone and bone marrow
An absolute Curie score of 0-2 prior to transplant has been shown to be more clinically prognostic than relative reduction in Curie score1-3
What’s next? Because she had an incomplete response to induction therapy, Maya is eligible for DANYELZA with GM-CSF4CT=computed tomography; FISH=fluorescence in situ hybridization; INSS=International Neuroblastoma Staging System; MIBG=meta-iodobenzylguanidine.
References: 1. Yanik GA, Parisi MT, Shulkin BL, et al. J Nucl Med. 2013;54(4):541-548. 2. Yanik GA, Parisi MT, Naranjo A, et al. J Nucl Med. 2018;59:502-508. 3. Streby KA, Parisi MT, Shulkin BL, et al. Pediatr Blood Cancer. 2023;e30418. https://doi.org/10.1002/pbc.30418. 4. DANYELZA® [package insert]. New York, NY: Y-mAbs Therapeutics, Inc; 2024.

Noah's background
3 years ago, Noah was diagnosed with INSS stage 4 high-risk neuroblastoma with disease present in his chest and metastatic disease in the bone
FISH testing showed no MYCN amplification
-
Noah underwent multimodal frontline therapy
5 cycles of chemotherapy
Surgical resection of the primary tumor
Consolidation treatment with high-dose chemotherapy and tandem ASCT
Maintenance treatment with immunotherapy
Relapse and disease characteristics
Noah was in remission for a little more than 1 year when he began experiencing abdominal pain with noticeable abdominal distension, nausea, and lethargy. He also developed a low-grade fever and discomfort in his legs that he described as “deep inside” pain
Soft tissue disease: CT scan showed a new abdominal mass
Bone and bone marrow disease MIBG scan and bone marrow aspiration revealed recurrent disease in the bone and new disease in the bone marrow
Curie score: 12
Status after relapse therapy: incomplete response with persistent disease in bone
Relapse therapy 5 cycles of chemoimmunotherapy; achieved a partial response
Bone marrow and metastatic soft tissue disease: resolved
Bone disease: reduced
Curie score: 6 (reduced by half from 12)
Considerations for next steps
Noah had an incomplete response to relapse therapy with persistent disease in the bone
What’s next? Because he had an incomplete response to relapse therapy, Noah is eligible for DANYELZA with GM-CSF1ASCT=autologous stem cell transplant; CT=computed tomography; FISH=fluorescence in situ hybridization; INSS=International Neuroblastoma Staging System; MIBG=meta-iodobenzylguanidine.
Reference: 1. DANYELZA® [package insert]. New York, NY: Y-mAbs Therapeutics, Inc; 2024.

A little more than 5 years ago, Kai was diagnosed with INSS stage 4 high-risk neuroblastoma with disease present in his abdomen and lymph nodes
FISH testing showed MYCN amplification
-
Kai underwent multimodal frontline therapy
5 cycles of induction therapy and surgical resection of the primary tumor
Consolidation treatment with high-dose chemotherapy followed by tandem ASCT and radiation therapy
Maintenance treatment including 5 cycles of an anti-GD2 antibody treatment
Relapse and disease characteristics
Relapse symptoms: Kai was in remission for just over 3 years when he began limping and complaining of leg and back pain. His parents noticed that he seemed unusually tired, with dark circles under his eyes, loss of appetite, and soon after, he developed a low-grade fever
Soft tissue disease: CT scan showed new tumors in the abdomen and chest
Bone and bone marrow disease MIBG scan and bone marrow aspiration revealed bone and bone marrow involvement
Curie score: 17
Status after relapse therapy: incomplete response with persistent disease in bone and bone marrow
Relapse therapy: 5 cycles of chemoimmunotherapy with anti-GD2 immunotherapy
Soft tissue disease: complete clearance of metastatic tumors
Bone and bone marrow disease: partial reduction
Curie score: 8 (reduced by more than half from 17)
Considerations for next steps
Kai has residual disease in both bone and bone marrow
Kai has received prior anti-GD2 therapy as part of his treatment regimen
What’s next? Because he had an incomplete response to relapse therapy with persistent disease in the bone and bone marrow, Kai is eligible for DANYELZA with GM-CSF1ASCT=autologous stem cell transplant; CT=computed tomography; FISH=fluorescence in situ hybridization; INSS=International Neuroblastoma Staging System; MIBG=meta-iodobenzylguanidine.
Reference: 1. DANYELZA® [package insert]. New York, NY: Y-mAbs Therapeutics, Inc; 2024.
Watch an Expert
DANYELZA Video Series
A physician’s insights on DANYELZA
Watch all 9 chapters. Explore important information about the efficacy and safety of DANYELZA with David Dickens, MD, FAAP, a pediatric hematologist-oncologist with over 20 years of experience.
This program is sponsored by Y-mAbs Therapeutics. Dr. Dickens received compensation from Y-mAbs Therapeutics for his participation in this video series.
-
1. High-risk neuroblastoma with bone and/or bone marrow disease

-
2. DANYELZA clinical studies: design and patient population

-
3. DANYELZA efficacy results in registrational studies

-
4. DANYELZA efficacy results in Study 201 pre-specified interim analysis, patients with an incomplete response to induction

-
5. DANYELZA efficacy results in Study 201 pre-specified interim analysis, patients with an incomplete response to relapse therapy

-
6. DANYELZA efficacy results in Study 201 pre-specified interim analysis, patients with prior anti-GD2 therapy

-
7. DANYELZA MOA

-
8. DANYELZA safety

-
9. DANYELZA dosing and administration

Video Chapter 1: High-risk neuroblastoma with bone and/or bone marrow disease
[PRESENTER: David Dickens, MD, FAAP, Pediatric Hematologist-Oncologist. Dr. Dickens received compensation from Y-mAbs Therapeutics for his participation in this video.]
DR. DICKENS: Hello, I’m David Dickens, a pediatric oncologist for over 20 years. In this video, I will address high-risk neuroblastoma with bone and/or bone marrow disease.
Neuroblastoma is the most common pediatric extracranial solid tumor.1 Half of neuroblastoma cases are classified as high risk, which typically involves metastatic disease.2,3 Bone and bone marrow are the most common sites of metastatic neuroblastoma.4,5 In children presenting with metastatic disease, 70% of metastases involve bone marrow and 55% involve cortical bone.4
Despite advances in care, response to high-risk neuroblastoma treatment remains poor, with approximately 2/3 of patients not obtaining a complete metastatic response to induction therapy.6 In other words, some patients are refractory to induction therapy, meaning they achieve a partial response, a minor response, or stable disease following treatment. Furthermore, despite intensive multimodal frontline treatment, 2 in 5 patients eventually relapse.7
So, what are the treatment goals for these patients? When high-risk neuroblastoma involves disease in the bone and/or bone marrow, reducing or eliminating that disease is a goal.2 Monitoring this goal involves assessing disease in the bone and bone marrow, which requires both biopsy and imaging.2 Some details are presented here.
[TEXT ON SCREEN: Bilateral aspirates, bilateral trephine biopsies detect and quantify NB cells in bone marrow; 123I-MIBG imaging detects metastases in bone for MIBG-avid tumors; FDG-PET, PET/CT scans for MIBG-nonavid tumors.]
DR. DICKENS: Note that MIBG imaging is widely used as a diagnostic and prognostic indicator for high-risk neuroblastoma.8 Specifically, MIBG imaging is used to quantify metastatic disease in the bone and soft tissue, generating a Curie score.2,8-10
To determine Curie score, the body is subdivided into 10 regions: 9 skeletal regions and 1 soft tissue region.8 Each region is designated a score of 0-3 points, with a maximum collective score of 30 points—the higher the score, the greater the extent of disease. Most of the score—up to 27 of the 30 points—is allocated to bone disease, with the remaining 3 potential points allocated to soft tissue.8
[VISUAL ON SCREEN: Diagram of a child’s body subdivided into 9 skeletal regions.]
Just as higher Curie scores reflect greater extent of disease, lower scores reflect less disease, with a score of 0 representing no disease in the bone or soft tissue.
Tracking Curie score throughout treatment allows you to monitor the disease course and treatment response. Importantly, an absolute Curie score of 0-2 prior to transplant has been shown to be more clinically prognostic than relative reduction in Curie score.8-10
Again, an incomplete response to treatment signifies residual disease, and if that disease is in the bone or soft tissue, the Curie score will be above 0.
I hope you found this video informative. To learn more about DANYELZA, you can watch the other chapters in this video series and visit danyelzahcp.com. Now, please take a moment to view the Important Safety Information for DANYELZA. Thank you.
References: 1. Smith V, Foster J. Children (Basel). 2018;5(9):114. 2. Park JR, Bagatell R, Cohn SL, et al. J Clin Oncol. 2017;35(22):2580-2587. 3. Irwin MS, Naranjo A, Zhang FF, et al. J Clin Oncol. 2021;39:3229-3241. 4. DuBois SG, Kalika Y, Lukens JN, et al. J Pediatr Hematol Oncol. 1999;21(3):181-189. 5. Sharp SE, Trout AT, Weiss BD, et al. Radiographics. 2016;36:258-278. 6. Garaventa A, Poetschger U, Valteau-Couanet D, et al. J Clin Oncol. 2021;39(23):2552-2563. 7. Pinto N, Naranjo A, Hibbitts E, et al. Eur J Cancer. 2019;112:66-79. 8. Yanik GA, Parisi MT, Naranjo A, et al. J Nucl Med. 2018;59:502-508. 9. Yanik GA, Parisi MT, Shulkin BL, et al. J Nucl Med. 2013;54(4):541-548. 10. Streby KA, Parisi MT, Shulkin BL, et al. Pediatr Blood Cancer. 2023;e30418. https://doi.org/10.1002/pbc.30418.
Video Chapter 2: DANYELZA clinical studies: design and patient population
[PRESENTER: David Dickens, MD, FAAP, Pediatric Hematologist-Oncologist. Dr. Dickens received compensation from Y-mAbs Therapeutics for his participation in this video.]
DR. DICKENS: Hello, I’m David Dickens, a pediatric oncologist for over 20 years. In this video, I’ll address the DANYELZA clinical studies.
DANYELZA is the only FDA-approved therapy for high-risk neuroblastoma in the bone and/or bone marrow when response to induction therapy or when response to relapse therapy is incomplete.1 Note that “incomplete response” is defined as a partial response, minor response, or stable disease to prior therapy. Treatment with DANYELZA is backed by more than a decade of clinical trial experience and was approved by the FDA in 2020.2
Excluded from the studies were patients with actively progressing disease and/or evaluable neuroblastoma outside of the bone and bone marrow.1
Study 12-230 was a phase 1/2, open-label, single-arm, single-center trial with a total of 72 participants.1 The safety analysis included all 72 patients who started an infusion of DANYELZA. The efficacy analysis included only patients with evaluable disease in the bone and/or bone marrow at baseline, which was 38 patients.
The second study, Study 201, is an ongoing phase 2, open-label, single-arm, global, multicenter trial.1 The initial analysis included 25 patients. All 25 patients who started an infusion were included in the safety analysis, and 22 of the 25 patients had evaluable disease in the bone and/or bone marrow at baseline and were included in the efficacy analysis.
A subsequent pre-specified interim analysis for Study 201 was conducted, for which additional patients were recruited, bringing the total number of participants up from 25 to 74.3 Trial sites are in the US, Canada, Denmark, Germany, Italy, Spain, and Hong Kong. Again, all patients who started an infusion were included in the safety analysis, and 52 of the 74 patients were included in the efficacy analysis.
The primary endpoint in both studies was overall response rate. Secondary endpoints included duration of response, complete response, and safety.1
Now let’s take a look at some baseline patient and disease characteristics in the DANYELZA with GM-CSF trials.
[VISUAL ON SCREEN: Table showing baseline patient and disease characteristics in Study 12-230, the Study 201 initial analysis, and the Study 201 pre-specified interim analysis.]
DR. DICKENS: The table shown breaks out the information by study, delineating patients included in the efficacy analysis for Study 12-230, the initial analysis of Study 201, and the pre-specified interim analysis of Study 201.1,3
As you can see, the table presents details on the patients’ type of disease, site of disease, and prior treatments. For instance, both refractory and relapsed patients were included in the trials, and many patients had poor prognostic factors, such as MYCN amplification and INSS stage 4 disease. Some patients had disease in bone only, some in bone marrow only, and some in both bone and bone marrow. The majority of patients had prior surgery and chemotherapy. Patients with prior radiation, stem cell transplant, and anti-GD2 antibody treatment were also included.1,3
The trials’ efficacy results are discussed in chapters 3-6. Feel free to pause the video if you’d like to take a few moments to review this information.
I hope you found this video informative. To learn more about DANYELZA, you can watch the other chapters in this video series and visit danyelzahcp.com. Now, please take a moment to view the Important Safety Information for DANYELZA. Thank you.
References: 1. DANYELZA® [package insert]. New York, NY: Y-mAbs Therapeutics, Inc.; 2024. 2. NIH US National Library of Medicine. https://clinicaltrials.gov/ct2/show/NCT01419834?term=NCT01419834&draw=2&rank=1. Accessed April 22, 2024. 3. Data on file. Y-mAbs Therapeutics, Inc.
Video Chapter 3: DANYELZA efficacy results in registrational studies
[PRESENTER: David Dickens, MD, FAAP, Pediatric Hematologist-Oncologist. Dr. Dickens received compensation from Y-mAbs Therapeutics for his participation in this video.]
DR. DICKENS: Hello, I’m David Dickens, a pediatric oncologist for over 20 years. In this video, I’ll address efficacy data from the DANYELZA with GM-CSF registrational studies.
As a reminder, DANYELZA may be considered for high-risk neuroblastoma patients with disease in the bone and/or bone marrow who have an incomplete response to induction therapy or who have an incomplete response to relapse therapy.1 Note that “incomplete response” is defined as a partial response, minor response, or stable disease to prior therapy.
There were 2 DANYELZA with GM-CSF registrational studies: Study 12-230 and Study 201.1 Details about the study designs and baseline patient and disease characteristics are described in chapter 2. Study 12-230 was a phase 1/2 trial with 38 patients included in the efficacy analysis. Study 201 is an ongoing phase 2 trial that had 22 patients in its initial efficacy analysis. A pre-specified interim analysis for Study 201 was conducted, and results from that interim analysis are presented in chapters 4 through 6.
For both studies, the primary endpoint was overall response rate, or ORR. ORR was defined as a complete response or partial response according to the INRC 2017 and confirmed by at least 1 subsequent assessment. Responses were evaluated by independent review.1
Now to the results. In Study 12-230 and the Study 201 initial analysis, more than 1/3 of patients responded and more than 1/4 of patients achieved a complete response with DANYELZA with GM-CSF.1
[VISUAL ON SCREEN: Bar graph showing overall response rate, complete response, and partial response data from Study 12-230.]
DR. DICKENS: As you can see in this bar graph from Study 12-230, 34% of patients responded and 26% achieved a complete response.1
[VISUAL ON SCREEN: Bar graph showing overall response rate, complete response, and partial response data from the Study 201 initial analysis.]
DR. DICKENS: This bar graph shows the results from the Study 201 initial analysis, in which 45% of patients responded and 36% achieved a complete response. The median duration of response was 6.2 months.1
To learn more about DANYELZA efficacy, including results of the Study 201 pre-specified interim analysis, please refer to chapters 4-6.
I hope you found this video informative. To learn more about DANYELZA, you can watch the other chapters in this video series and visit danyelzahcp.com. Now, please take a moment to view the Important Safety Information for DANYELZA. Thank you.
References: 1. DANYELZA® [package insert]. New York, NY: Y-mAbs Therapeutics, Inc.; 2024.
Video Chapter 4: DANYELZA efficacy results in Study 201 pre-specified interim analysis, patients with an incomplete response to induction
[PRESENTER: David Dickens, MD, FAAP, Pediatric Hematologist-Oncologist. Dr. Dickens received compensation from Y-mAbs Therapeutics for his participation in this video.]
DR. DICKENS: Hello, I’m David Dickens, a pediatric oncologist for over 20 years. In this video, I’ll address responses seen in the DANYELZA with GM-CSF registrational studies and then focus on results from the Study 201 pre-specified interim analysis. Specifically, I’ll present the results in all efficacy-evaluable patients and in those with a prior incomplete response to induction therapy.
As a reminder, DANYELZA may be considered for high-risk neuroblastoma patients with disease in the bone and/or bone marrow who have an incomplete response to induction therapy or who have an incomplete response to relapse therapy.1 Note that “incomplete response” is defined as a partial response, minor response, or stable disease to prior therapy.
There were 2 DANYELZA with GM-CSF registrational studies, which are described in detail in chapter 2.1 Study 12-230 was a phase 1/2 trial with 38 patients included in the efficacy analysis. Study 201 is a phase 2 trial that had 22 patients in its initial efficacy analysis1; a pre-specified interim analysis for Study 201 was conducted, for which additional patients were recruited, bringing the number of patients in the efficacy analysis to 52.2
The primary endpoint was overall response rate, or ORR. ORR was defined as a complete response or partial response according to the INRC 2017 and confirmed by at least 1 subsequent assessment.1
In Study 12-230 and the initial analysis of Study 201—in other words, the registrational studies—more than 1/3 of patients responded and more than 1/4 achieved a complete response.1
[VISUAL ON SCREEN: Bar graph showing overall response rate, complete response, and partial response data from the Study 201 pre-specified interim analysis.]
DR. DICKENS: Now let’s look at results from the Study 201 interim analysis. Shown here are the overall, complete, and partial responses for all 52 patients in the efficacy analysis.2 With a median follow-up of 5.9 months, 40% of patients responded to DANYELZA with GM-CSF, and 29% achieved a complete response. The remaining 11% of responders achieved a partial response.
For these responding patients, the median duration of response was not estimable because responses were ongoing or patients were censored at the time of data cutoff.2
[VISUAL ON SCREEN: Bar graph showing overall response, partial response, and complete response data from the Study 201 pre-specified interim analysis in patients with incomplete response to induction.]
DR. DICKENS: Next, let’s review response rates in the pre-specified subgroup of patients with an incomplete response to induction therapy. As a reminder from chapter 2, which includes baseline characteristics, half the efficacy-evaluable patients in the Study 201 interim analysis fell into this category.2
Shown here are the overall, complete, and partial responses for these patients. As you can see, 46% responded to DANYELZA with GM-CSF, and 31% achieved a complete response. The remaining 15% of responders achieved a partial response.2
[VISUAL ON SCREEN: Swimmer plot of patients in the Study 201 pre-specified interim analysis with incomplete response to induction.]
DR. DICKENS: In the swimmer plot, you see patient-level data of patients who had an incomplete response to induction therapy.2
The plot shows responses and Curie scores at baseline and at subsequent points following initiation of treatment with DANYELZA plus GM-CSF.
The colors within the bars correspond to the response, with gray reflecting that no assessment was performed and darkening shades of blue reflecting deeper responses. The letters represent an assessment timepoint and a dark square represents progressive disease.
The numbers to the right of the bars are each patient’s Curie scores during treatment, and they are plotted in the bars at the times they were assessed. Curie score assessments were performed at baseline, at first assessment of response, and at each subsequent assessment of response.
The baseline Curie score, which is the second column of numbers, is the Curie score assessed before the first administration of DANYELZA with GM-CSF.
Feel free to pause the video if you’d like to take a few moments to review this information.
To learn more about Curie score in high-risk neuroblastoma, please refer to chapter 1.
To learn about results in the subgroup of patients in the Study 201 interim analysis with an incomplete response to relapse therapy, please refer to chapter 5.
I hope you found this video informative. To learn more about DANYELZA, you can watch the other chapters in this video series and visit danyelzahcp.com. Now, please take a moment to view the Important Safety Information for DANYELZA. Thank you.
References: 1. DANYELZA® [package insert]. New York, NY: Y-mAbs Therapeutics, Inc.; 2024. 2. Data on file. Y-mAbs Therapeutics, Inc.
Video Chapter 5: DANYELZA efficacy results in Study 201 pre-specified interim analysis, patients with an incomplete response to relapse therapy
[PRESENTER: David Dickens, MD, FAAP, Pediatric Hematologist-Oncologist. Dr. Dickens received compensation from Y-mAbs Therapeutics for his participation in this video.]
DR. DICKENS: Hello, I’m David Dickens, a pediatric oncologist for over 20 years. In this video, I’ll address responses seen in the DANYELZA with GM-CSF registrational studies and then focus on results from the Study 201 pre-specified interim analysis. Specifically, I’ll present the results in all efficacy-evaluable patients and in those with an incomplete response to relapse therapy.
As a reminder, DANYELZA may be considered for patients with high-risk neuroblastoma in the bone and/or bone marrow who have an incomplete response to induction therapy or who have an incomplete response to relapse therapy.1 Note that “incomplete response” is defined as a partial response, minor response, or stable disease to prior therapy.
There were 2 DANYELZA with GM-CSF registrational studies, which are described in detail in chapter 2.1 Study 12-230 was a phase 1/2 trial with 38 patients included in the efficacy analysis. Study 201 is a phase 2 trial that had 22 patients in its initial efficacy analysis1; a pre-specified interim analysis for Study 201 was conducted, for which additional patients were recruited, bringing the number of patients in the efficacy analysis to 52.2
The study’s primary endpoint was overall response rate, or ORR. ORR was defined as a complete response or partial response according to the INRC 2017 and confirmed by at least 1 subsequent assessment.1
In Study 12-230 and the initial analysis of Study 201—in other words, the registrational studies—more than one-third of patients responded and more than one-fourth achieved a complete response.1
[VISUAL ON SCREEN: Bar graph showing overall response rate, complete response, and partial response data from the Study 201 pre-specified interim analysis.]
DR. DICKENS: Now let’s look at results from the Study 201 interim analysis. Shown here are the overall, complete, and partial responses for all patients in the efficacy analysis. With a median follow-up of 5.9 months, 40% of patients responded to DANYELZA with GM-CSF, and 29% achieved a complete response. The remaining 11% of responders achieved a partial response.2
For these responding patients, the median duration of response was not estimable because responses were ongoing or patients were censored at the time of data cutoff.
[VISUAL ON SCREEN: Bar graph showing overall response rate, complete response, and partial response data from the Study 201 pre-specified interim analysis in patients with incomplete response to relapse therapy.]
DR. DICKENS: Next, let’s review response rates in the pre-specified subgroup of patients with a prior incomplete response to relapse therapy. As a reminder from chapter 2, which includes baseline characteristics, half the patients in the Study 201 interim efficacy analysis fell into this category.2
Shown here are the overall, complete, and partial responses for these patients. As you can see, 35% responded to DANYELZA with GM-CSF, and 27% achieved a complete response. The remaining 8% of responders achieved a partial response.2
[VISUAL ON SCREEN: Swimmer plot of patients in the Study 201 pre-specified interim analysis with incomplete response to relapse therapy.]
DR. DICKENS: In the swimmer plot, you see patient-level data of patients who’d had an incomplete response to relapse therapy.2
The plot shows responses and Curie scores at baseline and at subsequent points following initiation of treatment with DANYELZA plus GM-CSF.
The colors within the bars correspond to the response, with gray reflecting that no assessment was performed and darkening shades of purple reflecting deeper responses. The letters represent an assessment timepoint and a dark square represents progressive disease.
The numbers to the right of the bars are each patient’s Curie scores during treatment, and they are plotted in the bars at the times they were assessed. Curie score assessments were performed at baseline, at first assessment of response, and at each subsequent assessment of response.
The baseline Curie score, which is the second column of numbers, is the Curie score assessed before the first administration of DANYELZA with GM-CSF.
Feel free to pause the video if you’d like to take a few moments to review this information.
For more information about Curie score in high-risk neuroblastoma, please refer to chapter 1. To learn about results in the subgroup of patients in the Study 201 interim analysis with an incomplete response to induction therapy, please refer to chapter 4. To learn about results in the subgroup of patients in the Study 201 interim analysis with prior anti-GD2 treatment, please refer to chapter 6.
I hope you found this video informative. To learn more about DANYELZA, you can watch the other chapters in this video series and visit danyelzahcp.com. Now, please take a moment to view the Important Safety Information for DANYELZA. Thank you.
References: 1. DANYELZA® [package insert]. New York, NY: Y-mAbs Therapeutics, Inc.; 2024. 2. Data on file. Y-mAbs Therapeutics, Inc.
Video Chapter 6: DANYELZA efficacy results in Study 201 pre-specified interim analysis, patients with prior anti-GD2 therapy
[PRESENTER: David Dickens, MD, FAAP, Pediatric Hematologist-Oncologist. Dr. Dickens received compensation from Y-mAbs Therapeutics for his participation in this video.]
DR. DICKENS: Hello, I’m David Dickens, a pediatric oncologist for over 20 years. In this video, I’ll review responses seen in the DANYELZA with GM-CSF registrational studies and then focus on results from the Study 201 pre-specified interim analysis. Specifically, I’ll present the results in all efficacy-evaluable patients, those with and without prior anti-GD2 therapy, and those who developed anti-drug antibodies during treatment with DANYELZA plus GM-CSF.
As a reminder, DANYELZA may be considered for patients with high-risk neuroblastoma in the bone and/or bone marrow who have an incomplete response to induction therapy or who have an incomplete response to relapse therapy.1 Note that “incomplete response” is defined as a partial response, minor response, or stable disease to prior therapy.1
There were 2 DANYELZA with GM-CSF registrational studies, which are described in detail in chapter 2.1 Study 12-230 was a phase 1/2 trial with 38 patients included in the efficacy analysis. Study 201 is a phase 2 trial that had 22 patients in its initial efficacy analysis1; a pre-specified interim analysis for Study 201 was conducted, for which additional patients were recruited, bringing the number of patients in the efficacy analysis to 52.2
[VISUAL ON SCREEN: Table showing baseline patient and disease characteristics in Study 12-230, the Study 201 initial analysis, and the Study 201 pre-specified interim analysis.]
DR. DICKENS: Shown here are baseline patient and disease characteristics in the DANYELZA with GM-CSF trials.1,2 The table breaks out the information by study and includes details about disease type, disease site, and prior treatments. One of those prior treatments is anti-GD2 antibody treatment. As I’ll be focusing on the Study 201 pre-specified interim analysis, you can draw your attention to the bottom row, last column and see that a quarter of patients had received prior anti-GD2 treatment.2
OK, let’s get to the results. The study’s primary endpoint was overall response rate, or ORR. ORR was defined as a complete response or partial response according to the INRC 2017 and confirmed by at least 1 subsequent assessment.1
In Study 12-230 and the initial analysis of Study 201—in other words, the registrational studies—more than 1/3 of patients responded and more than 1/4 achieved a complete response.1
[VISUAL ON SCREEN: Bar graph showing overall response rate, complete response, and partial response data from the Study 201 pre-specified interim analysis.]
DR. DICKENS: Now let’s look at results from the Study 201 pre-specified interim analysis. Shown here are the overall, complete, and partial responses for all patients in the efficacy analysis. With a median follow-up of 5.9 months, 40% of patients responded to DANYELZA with GM-CSF, and 29% achieved a complete response.2 The remaining 11% of responders achieved a partial response.
For these responding patients, the median duration of response was not estimable because responses were ongoing or patients were censored at the data cutoff.
Next, let’s review response rates in the pre-specified subgroups of patients with and without prior use of anti-GD2 therapy.
[VISUAL ON SCREEN: Bar graphs showing overall response rate, complete response, and partial response data from the Study 201 pre-specified interim analysis in patients by prior use of anti-GD2 antibody therapy.]
DR. DICKENS: Shown here are the overall, complete, and partial responses. As you can see, among patients with prior anti-GD2 therapy, 31% responded to DANYELZA with GM-CSF, and 23% achieved a complete response. The remaining 8% of responders achieved a partial response.2
As for patients without prior anti-GD2 therapy, 44% responded to DANYELZA with GM-CSF, and 31% achieved a complete response. The remaining 13% achieved a partial response.2
[VISUAL ON SCREEN: Bar graphs showing overall response rate, complete response, and partial response data from the Study 201 pre-specified interim analysis in patients who had developed anti-drug antibodies.]
DR. DICKENS: A quick note about anti-drug antibodies, or ADAs, and treatment with DANYELZA plus GM-CSF. Twenty-two percent of patients with positive ADA titers responded to DANYELZA with GM-CSF and 17% achieved a complete response.2
To learn more about results in other patient subgroups in the Study 201 interim analysis, please refer to chapters 4 and 5.
To learn about DANYELZA’s mechanism of action as a structurally distinct GD2-binding monoclonal antibody, refer to chapter 7.
I hope you found this video informative. To learn more about DANYELZA, you can watch the other chapters in this video series and visit danyelzahcp.com. Now, please take a moment to view the Important Safety Information for DANYELZA. Thank you.
References: 1. DANYELZA® [package insert]. New York, NY: Y-mAbs Therapeutics, Inc.; 2024. 2. Data on file. Y-mAbs Therapeutics, Inc.
Video Chapter 7: DANYELZA MOA
[PRESENTER: David Dickens, MD, FAAP, Pediatric Hematologist-Oncologist. Dr. Dickens received compensation from Y-mAbs Therapeutics for his participation in this video.]
DR. DICKENS: Hello, I’m David Dickens, a pediatric oncologist for over 20 years. In this video, I’ll address the mechanism of action of DANYELZA, the only FDA-approved therapy for high-risk neuroblastoma in the bone and/or bone marrow when response to induction or relapse therapy is incomplete.1 Note that “incomplete response” is defined as a partial response, minor response, or stable disease to prior therapy.
[VISUAL ON SCREEN: Illustration of the DANYELZA monoclonal antibody.]
DR. DICKENS: DANYELZA is a structurally distinct, humanized GD2-binding monoclonal antibody.1 This is important because GD2 is a disialoganglioside that is overexpressed on neuroblastoma cells.1
Structurally, DANYELZA’s framework is 92% human and 8% murine.2
In vitro studies showed that the binding affinity of DANYELZA to the GD2 receptor is approximately 10 times higher than that of approved chimeric anti-GD2 antibodies due to a slower off-rate.3 Any clinical significance and product comparisons of efficacy or safety should not be inferred.
[VISUAL ON SCREEN: Illustration of the DANYELZA mechanism of action.]
DR. DICKENS: DANYELZA prompts immune-mediated cell death via two pathways: the ADCC and CDC pathways.1
It was shown to bind GD2 and trigger immune-mediated cell death in vitro.1,3 As an immunotherapy, DANYELZA flags neuroblastoma cells for the immune system to target and destroy, which it does via ADCC and CDC in vitro.
I hope you found this video informative. To learn more about DANYELZA, you can watch the other chapters in this video series and visit danyelzahcp.com. Now, please take a moment to view the Important Safety Information for DANYELZA. Thank you.
References: 1. DANYELZA® [package insert]. New York, NY: Y-mAbs Therapeutics, Inc.; 2024. 2. Cheung N-KV, Guo H, Hu J, et al. Oncoimmunology. 2012;1(4):477-486. 3. Lisby S, Liebenberg N, Bukrinski J, et al. Presented at the SIOP virtual congress. Abstract #945. October 16, 2020.
Video Chapter 8: DANYELZA safety
[PRESENTER: David Dickens, MD, FAAP, Pediatric Hematologist-Oncologist. Dr. Dickens received compensation from Y-mAbs Therapeutics for his participation in this video.]
DR. DICKENS: Hello, I’m David Dickens, a pediatric oncologist for over 20 years. In this video, I’ll address DANYELZA safety, as established in clinical trials.
As a reminder, there were 2 DANYELZA with GM-CSF registrational studies, which are described in detail in chapter 2.1 Study 12-230 was a phase 1/2 trial with 72 patients. Study 201 is a phase 2 trial that had 25 patients in its initial analysis;1 subsequently, a pre-specified interim analysis for Study 201 was conducted, for which additional patients were recruited, bringing the number of patients to 74.2 In all three analyses, all patients who started an infusion of DANYELZA were included in the safety evaluation. I’ll be presenting information from all three analyses.
[VISUAL ON SCREEN: Table showing safety analysis of patients who received DANYELZA with GM-CSF.]
DR. DICKENS: Let’s begin by reviewing potential serious adverse reactions. Note that DANYELZA can cause serious infusion reactions, including hypotension, bronchospasm, hypoxia, and stridor, as well as severe neurotoxicity, including pain.1
The most common adverse reactions, occurring in at least 25% of patients, are shown here. It is important to familiarize yourself with these adverse reactions, so feel free to pause the video if you’d like to take a few moments to review this information.1,2
Now you can see the percentage of patients who were exposed to DANYELZA + GM-CSF for at least 6 months and at least 1 year across Study 12-230 and the Study 201 initial and interim analyses.1,2
Bear in mind that the DANYELZA Prescribing Information provides the full list of adverse reactions that occurred in Study 12-230 and the Study 201 initial analysis, as well as recommendations for management of adverse reactions. Chapter 9 goes into some detail about these management strategies.
[VISUAL ON SCREEN: Bar graphs showing serious adverse reactions in the DANYELZA clinical trials and permanent discontinuations.]
DR. DICKENS: Now in the DANYELZA + GM-CSF clinical studies, some adverse reactions led to treatment discontinuation. Across the 3 analyses, 32% to 45% of patients had a serious adverse reaction, and in 8% to 12% of patients, an adverse reaction led to treatment discontinuation.1,2
Overall, the DANYELZA safety profile in the Study 201 interim analysis was consistent with that in the initial analysis.1,2
Identified in this table are specific adverse reactions and the severity of those reactions that necessitate treatment discontinuation.1 For more details about the interventions that are recommended, please refer to the DANYELZA Prescribing Information or watch chapter 9.
[VISUAL ON SCREEN: Bar graph showing resolution of select Grade 3 or 4 adverse reactions in the Study 201 pre-specified interim analysis.]
DR. DICKENS: The last DANYELZA safety consideration that I’ll review is time to resolution of select Grade 3 or 4 adverse reactions in the Study 201 interim analysis. On your screen, you’ll see two bar charts—the one on the left shows time to resolution of Grade 3 pain, and the one on the right shows time to resolution of Grade 3 or 4 hypotension.2
As you can see, the majority of Grade 3 pain was resolved within an hour and 90% was resolved in ≤5 hours.2 Similarly, the majority of Grade 3 or 4 hypotension was resolved within an hour and 89% was resolved in ≤5 hours.2
To be considered “resolved,” the adverse reaction needed to resolve completely—in other words, diminish to the degree that it would no longer be on the grading scale, so the severity of these events may have decreased before completely resolved.
To learn more about strategies to manage adverse reactions, refer to chapter 9, which speaks to those approaches as part of the DANYELZA dosing and administration presentation.
I hope you found this video informative. To learn more about DANYELZA, you can watch the other chapters in this video series and visit danyelzahcp.com. Now, please take a moment to view the Important Safety Information for DANYELZA. Thank you.
References: 1. DANYELZA® [package insert]. New York, NY: Y-mAbs Therapeutics, Inc.; 2024. 2. Data on file. Y-mAbs Therapeutics, Inc.
Video Chapter 9: DANYELZA dosing and administration
[PRESENTER: David Dickens, MD, FAAP, Pediatric Hematologist-Oncologist. Dr. Dickens received compensation from Y-mAbs Therapeutics for his participation in this video.]
DR. DICKENS: Hello, I’m David Dickens, a pediatric oncologist for over 20 years. In this video, I’ll address DANYELZA dosing and administration.
As a reminder, DANYELZA may be considered for patients with high-risk neuroblastoma in the bone and/or bone marrow who have an incomplete response to induction therapy or who have an incomplete response to relapse therapy.1 Note that “incomplete response” is defined as a partial response, minor response, or stable disease to prior therapy.
DANYELZA can offer the flexibility of inpatient or outpatient administration, at the treating physician’s discretion. In the Study 201 pre-specified interim analysis, more than 90% of infusions were given in an outpatient setting.2
The recommended dosage of DANYELZA is 3 mg/kg/day (up to 150 mg/day) given on Days 1, 3, and 5 of each treatment cycle.1 DANYELZA is administered as an intravenous infusion after dilution in combination with GM-CSF, which is given subcutaneously. This timeline shows you when GM-CSF and DANYELZA are given.1
In each cycle, GM-CSF begins five days before the first DANYELZA infusion and is dosed at 250 μg/m2/day. On Days 1-5 of each cycle, GM-CSF is continued, but its dose is increased to 500 μg/m2/day. So, in total, GM-CSF will be administered for 10 days during each cycle.1
For the very first infusion of DANYELZA—in other words, Cycle 1, Day 1—the infusion is given over 60 minutes. Thereafter, infusions take 30-60 minutes, as tolerated.1
After each DANYELZA infusion, patients should be closely observed for at least 2 hours in a setting where cardiopulmonary resuscitation medication and equipment are available.
The recommended treatment course of DANYELZA + GM-CSF is to continue 28-day cycles until the patient achieves a complete response or a partial response.1 Following that response, the 28-day cycle is repeated for five more cycles. At that point, at the physician’s discretion, treatment may switch to 8-week cycles and may be discontinued for disease progression or unacceptable toxicity.
DANYELZA with GM-CSF treatment involves premedication and supportive medication.1 To reduce the risk of infusion-related reactions and nausea or vomiting, IV corticosteroids are administered 120 to 30 minutes before the first DANYELZA infusion. They may also be given with subsequent infusions if a severe reaction occurred with the previous infusion or during the previous cycle. In addition, an antihistamine, an H2 antagonist, acetaminophen, and an antiemetic are administered 30 minutes before each infusion.
For potential pain, a 12-day course of gabapentin, or another prophylactic medication for neuropathic pain, is initiated five days before the first DANYELZA infusion in each cycle—this is the same day that GM-CSF is initiated.1 Sixty to 45 minutes before each infusion, oral opioids are administered, and additional IV opioids may be given during the infusion if breakthrough pain occurs. Finally, ketamine may be considered for pain that is not adequately controlled by opioids.1
For more details about management of adverse reactions, please refer to the DANYELZA full Prescribing Information.
I hope you found this video informative. To learn more about DANYELZA, you can watch the other chapters in this video series and visit danyelzahcp.com. Now, please take a moment to view the Important Safety Information for DANYELZA. Thank you.
References: 1. DANYELZA® [package insert]. New York, NY: Y-mAbs Therapeutics, Inc.; 2024. 2. Data on file. Y-mAbs Therapeutics, Inc.
A nurse’s insights on DANYELZA
Explore important considerations for administration and use of DANYELZA with Rita Secola, PhD, RN, CPON, NEA-BC, FAAN.
This program is sponsored by Y-mAbs Therapeutics. Rita Secola received compensation from Y-mAbs Therapeutics for her participation in this video series.
-
1. What is DANYELZA?

-
2. Clinical efficacy

-
3. Dosing, administration, and treatment course

-
4. Managing adverse events

-
5. Counseling patients and their caregivers

Video Chapter 1: What is DANYELZA?
[PRESENTER: Rita Secola, PhD, RN, CPON, NEA-BC, FAAN. Dr. Secola is speaking on behalf of Y-mAbs Therapeutics, the sponsor of this video series. Dr. Secola received compensation from Y-mAbs Therapeutics for her participation in this video.]
DR. SECOLA: Hello. I’m Rita Secola, Executive Director and Associate Chief Nursing Officer at Children’s Hospital Los Angeles. I am a Certified Pediatric Oncology Nurse with 40 years’ experience. In this series of videos, I’ll address DANYELZA dosing and administration.
[VISUAL ON SCREEN: The DANYELZA antibody.]
DR. SECOLA: DANYELZA is a structurally distinct, humanized GD2-binding monoclonal antibody.1,3 This is important because GD2 is an antigen that is overexpressed on neuroblastoma cells.1
Structurally, DANYELZA’s framework is 92% human and 8% murine.2
In vitro studies showed that the binding affinity of DANYELZA to the GD2 receptor is approximately 10 times higher than that of approved chimeric anti-GD2 antibodies due to a slower off-rate.3 Any clinical significance and product comparisons of efficacy or safety should not be inferred.
DANYELZA may be considered for patients with high-risk neuroblastoma in the bone and/or bone marrow who have an incomplete response to induction therapy or who have an incomplete response to relapse therapy.1 Note that “incomplete response” is defined as a partial response, minor response, or stable disease to prior therapy.
DANYELZA is backed by more than 10 years of clinical trial experience4; it received accelerated approval in 2020.1 Since then, DANYELZA has been administered at more than 60 US healthcare institutions.5 That number continues to grow. You should also know: support is available for you and your patient through
Y-mAbs Connect. Getting your patient started on DANYELZA is a simple 3-step process:
- Step 1: Enroll your patient in Y-mAbs Connect. You can scan the QR code here, or go to the ymabsconnect.com website for an enrollment form
- Step 2: Once your patient’s coverage is confirmed, place the order of DANYELZA
- Step 3: Receive your DANYELZA order, which will be shipped directly to the infusion site location
In addition to information in this video series, downloadable resources are available on the DANYELZA HCP website. These include a preparation and administration guide, a billing and coding reference guide, and other clinical resources, which can be accessed at the URL shown here (https://danyelzahcp.com/access-resources/clinical-resources/) or by scanning the QR code.
I hope you found this video informative. To learn more about DANYELZA, you can watch the other chapters in this video series and visit danyelzahcp.com. Now, please take a moment to view the Indication and Important Safety Information for DANYELZA. Thank you.
References: 1. DANYELZA® [package insert]. New York, NY: Y-mAbs Therapeutics, Inc.; 2024. 2. Cheung N-KV, Guo H, Hu J, et al. Oncoimmunology. 2012;1(4):477-486. 3. Lisby S, Liebenberg N, Bukrinski J, et al. Presented at the SIOP virtual congress. Abstract #945. October 16, 2024. 4. NIH US National Library of Medicine. https://clinicaltrials.gov/ct2/show/NCT01419834?term=NCT01419834&draw=2&rank=1. Accessed April 22, 2024. 5. Data on file. Y-mAbs Therapeutics, Inc
Video Chapter 2: Clinical Efficacy
[PRESENTER: Rita Secola, PhD, RN, CPON, NEA-BC, FAAN. Dr. Secola is speaking on behalf of Y-mAbs Therapeutics, the sponsor of this video series. Dr. Secola received compensation from Y-mAbs Therapeutics for her participation in this video.]
DR. SECOLA: Hello. I’m Rita Secola, Executive Director and Associate Chief Nursing Officer at Children’s Hospital Los Angeles. I am a Certified Pediatric Oncology Nurse with 40 years’ experience. In this video, I’ll address the DANYELZA clinical studies
DANYELZA, with GM-CSF, was granted accelerated approval based on overall response rate and duration of response in two clinical studies: Study 12-230 and Study 201.1 The studies included patients 12 months or older with high-risk neuroblastoma in the bone and/or bone marrow but no soft tissue disease, and they had an incomplete response to induction or relapse therapy. All patients had received at least one prior systemic therapy to treat disease outside of the bone and/or bone marrow. Patients who had received prior anti-GD2 antibody therapy were permitted.2
The primary endpoint in both studies was overall response rate, or ORR. Secondary endpoints included duration of response, complete response, and safety.1
Study 12-230 was a phase 1/2, open-label, single-arm, single-center trial with a total of 72 participants.1 The safety analysis included all 72 patients who started an infusion of DANYELZA. The efficacy analysis included only patients with evaluable disease in the bone and/or bone marrow at baseline, which was 38 patients.
The second study, Study 201, is an ongoing phase 2, open-label, single-arm, global, multicenter trial.1 The initial analysis included 25 patients. All 25 patients who started an infusion were included in the safety analysis, and 22 of the 25 patients had evaluable disease in the bone and/or bone marrow at baseline and were included in the efficacy analysis.1
A subsequent pre-specified interim analysis for Study 201 was conducted, for which additional patients were recruited, bringing the total number of participants up from 25 to 74.2 Trial sites are in the US, Canada, Denmark, Germany, Italy, Spain, and Hong Kong. Again, all patients who started an infusion were included in the safety analysis, and 52 of the 74 patients were included in the efficacy analysis.2
In both trials, DANYELZA demonstrated efficacy in the primary endpoint of ORR. In Study 12-230, 34% of patients responded; 26% achieved a complete response.1
In the Study 201 initial analysis, 45% of patients responded; 36% achieved a complete response.1
In the Study 201 interim analysis, 40% of patients responded to DANYELZA with GM-CSF, and 29% achieved a complete response.2
Now, let’s review response rates in the pre-specified subgroups in Study 201. In the subgroup of patients with an incomplete response to induction therapy—by the way, half of the efficacy-evaluable patients fell into this category—46% responded to DANYELZA with GM-CSF, and 31% achieved a complete response.2
In the subgroup of patients with an incomplete response to relapse therapy, 35% overall responded; 27% achieved a complete response.2
Finally, in the subgroup of patients who had received anti-GD2 therapy prior to this study, 31% overall achieved a response; 23% were complete responses.2
[VISUAL ON SCREEN: Table of the most common adverse reactions (ARs) in DANYELZA Study 12-230 and Study 201 Initial and Interim Analyses (≥25% in either study)1,2]
DR. SECOLA: The most common adverse reactions, occurring in at least 25% of patients, are shown here. This table includes ARs in Study 12-230, Study 201 Initial Analysis, and Study 201 Pre-specified Interim Analysis.1,2 It is important to familiarize yourself with these adverse reactions, so feel free to pause the video if you’d like to take a few moments to review this information.
In addition to information in this video series, downloadable resources are available on the DANYELZA HCP website. These include a preparation and administration guide, a billing and coding reference guide, and other clinical resources, which can be accessed at the URL shown here (https://danyelzahcp.com/access-resources/clinical-resources/) or by scanning the QR code.
I hope you found this video informative. To learn more about DANYELZA, you can watch the other chapters in this video series and visit danyelzahcp.com. Now, please take a moment to view the Indication and Important Safety Information for DANYELZA. Thank you.
References: 1. DANYELZA® [package insert]. New York, NY: Y-mAbs Therapeutics, Inc.; 2024. 2. Data on file. Y-mAbsY-mAbs Therapeutics, Inc.
Video Chapter 3: Dosing, Administration, and Treatment Course
[PRESENTER: Rita Secola, PhD, RN, CPON, NEA-BC, FAAN. Dr. Secola is speaking on behalf of Y-mAbs Therapeutics, the sponsor of this video series. Dr. Secola received compensation from Y-mAbs Therapeutics for her participation in this video.]
DR. SECOLA: Hello. I’m Rita Secola, Executive Director and Associate Chief Nursing Officer at Children’s Hospital Los Angeles. I am a Certified Pediatric Oncology Nurse with 40 years’ experience. In this video, I’ll address the treatment course for DANYELZA and how to administer it.
DANYELZA offers the flexibility of administration in either the outpatient or inpatient setting—at the discretion of the treating physician—offering the possibility for the patient to return home after their infusion. In fact, in the clinical trials that led to approval, more than 90% of infusions were administered in outpatient settings.1
[VISUAL ON SCREEN: Timeline showing the administration of GM-CSF and DANYELZA.]
DR. SECOLA: DANYELZA is administered as an intravenous infusion after dilution in combination with GM-CSF, which is given subcutaneously. This timeline shows you when GM-CSF and DANYELZA are given within each 28-day cycle.2
In each cycle, GM-CSF administration begins five days before the first DANYELZA infusion and is dosed at 250 μg/m2/day. On Days 1-5 of each cycle, GM-CSF is continued daily, but its dose is increased to 500 μg/m2/day. So, in total, GM-CSF will be administered for 10 days during each cycle. Be sure to administer GM-CSF at least 1 hour before DANYELZA, which is infused on Days 1, 3, and 5 at 3 mg/kg/day.2
Refer to the GM-CSF Prescribing Information for additional recommended dosing information.
For the very first infusion of DANYELZA—Cycle 1, Day 1—DANYELZA is administered over 60 minutes. Thereafter, infusions take 30-60 minutes, as tolerated.2
After each DANYELZA infusion, patients should be closely observed for at least 2 hours in a setting where cardiopulmonary resuscitation medication and equipment are available.2
If a DANYELZA dose is missed, administer the missed dose by Day 10 of the following week.2
In this case, administer GM-CSF 500 μg/m2/day on the first day of the DANYELZA infusion, and on the day before and the day of the second and third infusions, for a total of 5 days with GM-CSF 500 μg/m2/day.2
The recommended treatment course of DANYELZA + GM-CSF is to continue 28-day cycles until the patient achieves a complete response or a partial response.2 Following that response, the 28-day cycle is repeated for five more cycles. At that point, at the physician’s discretion, treatment may switch to 8-week cycles.2 DANYELZA may be discontinued at any point for disease progression or unacceptable toxicity.2
[VISUAL ON SCREEN: Timeline showing the administration schedule for premedication and supportive medications for pain.]
DR. SECOLA: DANYELZA with GM-CSF treatment involves premedication and supportive medication.2 For potential pain, a 12-day course of gabapentin, or another prophylactic medication for neuropathic pain, is initiated five days before the first DANYELZA infusion in each cycle—this is the same day that GM-CSF is initiated.2 Sixty to 45 minutes before each infusion, oral opioids are administered, and additional IV opioids may be given during the infusion if breakthrough pain occurs. Finally, ketamine may be considered for pain that is not adequately controlled by opioids.2
[VISUAL ON SCREEN: Timeline showing the administration schedule for premedications for infusion-related reactions (IRRs) and nausea/vomiting]
DR. SECOLA: To reduce the risk of infusion-related reactions and nausea or vomiting, IV corticosteroids are administered 120 to 30 minutes before the first DANYELZA infusion. They may also be given with subsequent infusions if a severe reaction occurred with the previous infusion or during the previous cycle. In addition, an antihistamine, an H2 antagonist, acetaminophen, and an antiemetic are administered 30 minutes before each infusion.2
For more details about management of adverse reactions, please refer to the DANYELZA full Prescribing Information.
As I mentioned, patients should be monitored during—and for at least 2 hours after—the infusion. Monitor closely for infusion-related reactions, which may include hypotension, bronchospasm, hypoxia, and stridor.2
In Study 201 initial analysis, onset was generally within 24 hours of infusion, most often within 30 minutes of initiating infusion, and most frequently during the first infusion of DANYELZA in each cycle.2
Serious IRRs may require urgent intervention, including fluid resuscitation, administration of bronchodilators and corticosteroids, intensive care unit admission, infusion rate reduction, or interruption of DANYELZA infusion.2
Blood pressure should be monitored during the DANYELZA infusion and daily on Days 1 through 8 of each DANYELZA cycle; patients should be evaluated for complications of hypertension.2
In the registrational trials, most hypertension events were observed on the day of the DANYELZA infusion, and occurred up to 9 days after the infusion.2
Patients should be monitored for symptoms of myocarditis, including chest pain, shortness of breath, or abnormal heart rhythms.2
Myocarditis has occurred in adolescent patients receiving DANYELZA in clinical trials and expanded access programs within days of receiving DANYELZA requiring drug interruption.2
Patients should be monitored for orthostatic hypotension; in patients with symptoms, monitor postural blood pressure prior to initiating treatment with DANYELZA and as clinically indicated with subsequent dosing.2
Severe cases requiring hospitalization have occurred; cases occurred within hours and up to 6 days of DANYELZA infusions in any cycle.2
Patients should be monitored for lab abnormalities, including cytopenia, changes in glucose, liver, and cardiac abnormalities.2
In clinical studies, the most common Grade 3 or 4 laboratory abnormalities (≥5% in either study) were decreased lymphocytes, decreased neutrophils, decreased hemoglobin, decreased platelet count, decreased potassium, increased alanine aminotransferase, decreased glucose, decreased calcium, decreased albumin, decreased sodium, and decreased phosphate.2
Detailed information for managing adverse events and infusion-related reactions can be learned by watching Chapter 4 in this video series.
In addition to information in this video series, downloadable resources are available on the DANYELZA HCP website. These include a preparation and administration guide, a coding and billing reference guide, and other clinical resources. These can be accessed at the URL shown here (https://danyelzahcp.com/access-resources/clinical-resources/) or by scanning the QR code.
I hope you found this video informative. To learn more about DANYELZA, you can watch the other chapters in this video series and visit danyelzahcp.com. Now, please take a moment to view the Indication and Important Safety Information for DANYELZA. Thank you.
References: 1. DANYELZA® [package insert]. New York, NY: Y-mAbs Therapeutics, Inc.; 2024. 2. Data on file. Y-mAbs Therapeutics, Inc.
Video Chapter 4: Managing Adverse Events
[PRESENTER: Rita Secola, PhD, RN, CPON, NEA-BC, FAAN. Dr. Secola is speaking on behalf of Y-mAbs Therapeutics, the sponsor of this video series. Dr. Secola received compensation from Y-mAbs Therapeutics for her participation in this video.]
DR. SECOLA: Hello. I’m Rita Secola, Executive Director and Associate Chief Nursing Officer at Children’s Hospital Los Angeles. I am a Certified Pediatric Oncology Nurse with 40 years’ experience. In this video, I’ll address how to manage adverse events and infusion-related reactions seen with DANYELZA.
[VISUAL ON SCREEN: Table showing common terminology criteria for adverse events, from Grade 1 to 5, with severity and descriptions of each Grade.]
DR. SECOLA: To begin, let’s familiarize ourselves with the common terminology for grading the severity of adverse events and infusion-related reactions. Grades go from 1—mild—to 5, which would result in death. Typically, Grade 3 and 4 adverse events will require dose modification.1
[VISUAL ON SCREEN: Table showing dose modifications for managing adverse reactions.]
DR. SECOLA: Now, I’ll review how to modify the dose or discontinue DANYELZA based on the different grades of specific adverse events. Shown here are dosage modifications for infusion-related reactions, or IRRs.
For Grade 2 IRRs, the infusion rate should be reduced to 50% of the previous rate and the patient should be monitored closely until they recover to Grade 1 or less.2 Once the patient has recovered, the infusion rate can be gradually increased to the rate prior to the event, as tolerated.2
For Grade 3 IRRs, immediately interrupt DANYELZA and monitor closely until the patient recovers to Grade 2 or less.2 Upon recovery, resume the infusion rate at 50% and gradually increase as tolerated.2
For Grade 3 IRRs that are not responsive to medical intervention (such as antihistamines, NSAIDs, narcotics, IV fluids), DANYELZA should be permanently discontinued.2
Permanently discontinue DANYELZA for any Grade 4 IRR or any Grade 3 or 4 anaphylaxis.
DANYELZA dosing should be modified to manage neurotoxicity according to the table shown here.
Treatment should be discontinued for2:
- Any Grade 3 pain that does not respond to maximum supportive measures
- Any-grade RPLS or transverse myelitis
- Motor neuropathy Grade 2 or higher
-
Sensory neuropathy Grade 3 or 4
- Grade 2 through 4 neurological disorders of the eye not resolved within 2 weeks
- Any subtotal or total vision loss
- Prolonged urinary retention that persists after opioid discontinuation
For Grade 2 or 3 myocarditis, withhold or reduce the dose, or permanently discontinue DANYELZA based on severity and duration of symptoms.2
For Grade 4 myocarditis, permanently discontinue DANYELZA.2
It is important to note that DANYELZA should not be given to patients with uncontrolled hypertension.2
For patients who develop hypertension during the DANYELZA infusion, withhold or pause the infusion until recovery to Grade 2 or below, then resume the infusion at 50% of the prior rate. If tolerated without recurrence of symptoms, gradually increase to the rate prior to onset of symptoms.2
Permanently discontinue DANYELZA for Grade 3 hypertension not responding to medical intervention or any Grade 4 hypertension.2
Now let’s look at how to manage hypotension. If a patient develops any-grade orthostatic hypotension, withhold DANYELZA until recovery to Grade 1 or better.2
If resolved within 1 week, restart DANYELZA at 50% of the prior dose; if tolerated without recurrence of symptoms, resume at the full recommended dose in subsequent cycles. Permanently discontinue DANYELZA if not resolved within a week.2
For any other adverse reactions, manage based on severity, as shown here.2
Permanently discontinue DANYELZA for Grade 4 reactions or Grade 3 ARs that are not resolved to Grade 2 or less within 2 weeks.2 Refer to the DANYELZA full Prescribing Information for additional guidance on managing these adverse reactions.
While we’ve just discussed strategies to manage adverse reactions, including premedication and dosage modifications, I’d like to point out the clinical trial experience of select adverse reaction resolutions.
[VISUAL ON SCREEN: Charts showing resolution of select Grade 3 or Grade 4 adverse reactions in the Study 201 Pre-specified Interim Analysis.]
DR. SECOLA: Shown here are data on time to resolution of Grade 3 pain and Grade 3 or 4 hypotension from Study 201 Pre-specified Interim Analysis.3
The bar chart on the left shows that, in about half of cases, Grade 3 pain was resolved within an hour; within 5 hours, 90% of Grade 3 pain had resolved.3
Similarly, 70% of Grade 3 or 4 hypotension was resolved within an hour, and by 5 hours, almost all was resolved.3
Note that to be considered “resolved,” the adverse reaction needed to be resolved completely—in other words, it would no longer be on the grading scale, so the severity of these events may have diminished before being completely resolved.
In addition to information in this video series, downloadable resources are available on the DANYELZA HCP website. These include a preparation and administration guide, a billing and coding reference guide, and other clinical resources. These can be accessed at the URL shown here (https://danyelzahcp.com/access-resources/clinical-resources/) or by scanning the QR code.
I hope you found this video informative. To learn more about DANYELZA, you can watch the other chapters in this video series and visit danyelzahcp.com. Now, please take a moment to view the Indication and Important Safety Information for DANYELZA. Thank you.
References: 1. Common terminology criteria for adverse events (CTCAE). Version 5.0. Published 11/27/2017. 2. DANYELZA® [package insert]. New York, NY: Y-mAbs Therapeutics, Inc.; 2024. 3.. Data on file. Y-mAbs Therapeutics, Inc.
Video Chapter 5: Counseling Patients and Their Caregivers
PRESENTER: Rita Secola, PhD, RN, CPON, NEA-BC, FAAN. Dr. Secola is speaking on behalf of Y-mAbs Therapeutics, the sponsor of this video series. Dr. Secola received compensation from Y-mAbs Therapeutics for her participation in this video.
DR. SECOLA: Hello. I’m Rita Secola, Executive Director and Associate Chief Nursing Officer at Children’s Hospital Los Angeles. I am a Certified Pediatric Oncology Nurse with 40 years’ experience.
In Chapter 4, I discussed adverse events seen with DANYELZA and how to manage them. As part of your monitoring for adverse events, it’s important to have patients and their caregivers be vigilant.
Advise them to immediately contact their HCP for any new or worsening adverse events that occur during or after the DANYELZA infusions, including the following1:
- Facial or lip swelling, itching, rash, trouble breathing, cough/wheezing, or dizziness. These can be signs of infusion-related reactions
- Severe pain: Including pain in the belly, bone, neck, legs, or arms
- Weakness in arms or legs; bladder and bowel problems; pain in back, legs, or stomach; numbness, tingling, or burning sensation, all of which can be signs of inflammation of the spinal cord
- Severe headache, vision changes, confusion or disorientation, decreased alertness, difficulty speaking, weakness in arms or legs, or seizures
- Unequal pupil size, blurred vision, mydriasis, visual impairment, or photophobia
- Problems urinating or emptying the bladder
- Chest pain, shortness of breath, or abnormal heart rhythms
- Dizziness, lightheadedness, or fainting, especially when standing after sitting or lying down
Again, it is important you advise patients’ caregivers to immediately contact their HCP for any new or worsening adverse events.
In addition to information in this video series, downloadable resources are available on the DANYELZA HCP website. These include a preparation and administration guide, a billing and coding reference guide, and other clinical resources. These can be accessed at the URL shown here (https://danyelzahcp.com/access-resources/clinical-resources/) or by scanning the QR code.
I hope you found this video informative. To learn more about DANYELZA, you can watch the other chapters in this video series and visit danyelzahcp.com. Now, please take a moment to view the Indication and Important Safety Information for DANYELZA. Thank you.
References: 1. DANYELZA® [package insert]. New York, NY: Y-mAbs Therapeutics, Inc.; 2024. 2. Data on file. Y-mAbs Therapeutics, Inc. 3. Yanik GA, Parisi MT, Naranjo A, et al. J Nucl Med. 2018;59:502-508.
DANYELZA Downloadable Resources
Starting your patients on DANYELZA

Clinical Overview
An informative resource about DANYELZA with GM-CSF clinical studies, efficacy, safety, and dosing and administration
Download
Preparation and Administration Guide
A practical resource that outlines the process of ordering, storing, preparing, and administering DANYELZA, as well as managing potential adverse reactions
Download
Considerations for Administration and Use
Information to optimize the administration and use of DANYELZA
Download
Dosage Modifications and Adverse Event Management Guide
A guide to counseling, adverse reactions monitoring, and dosage modifications
Download