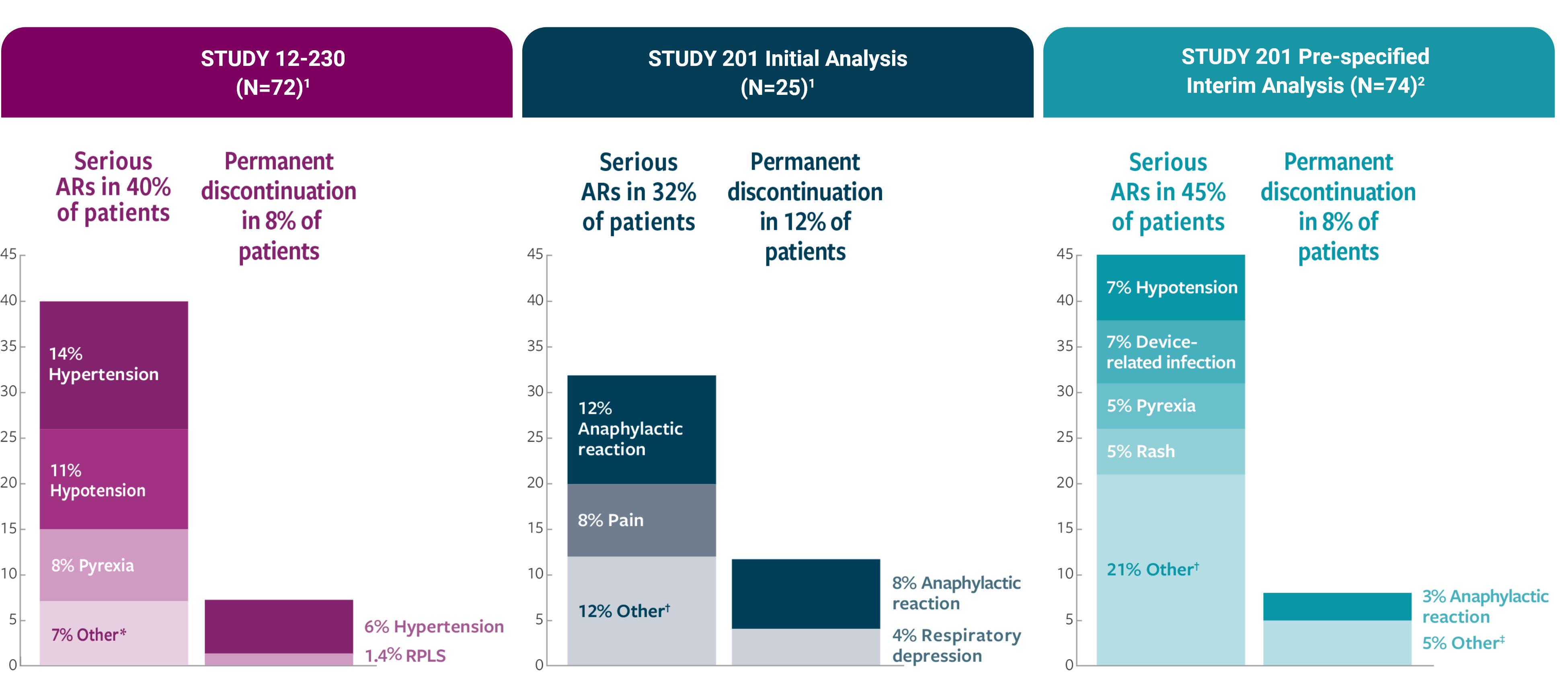
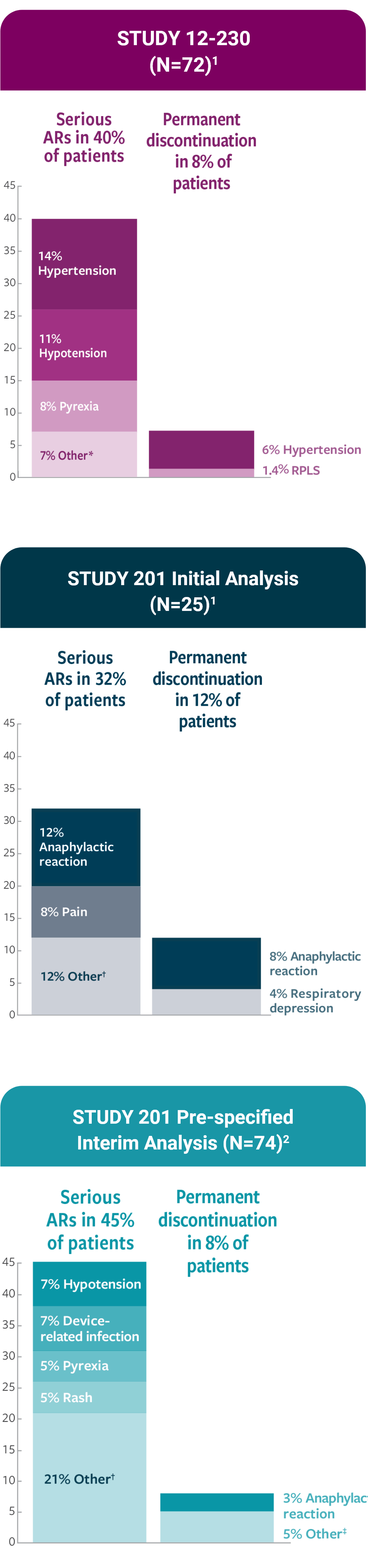
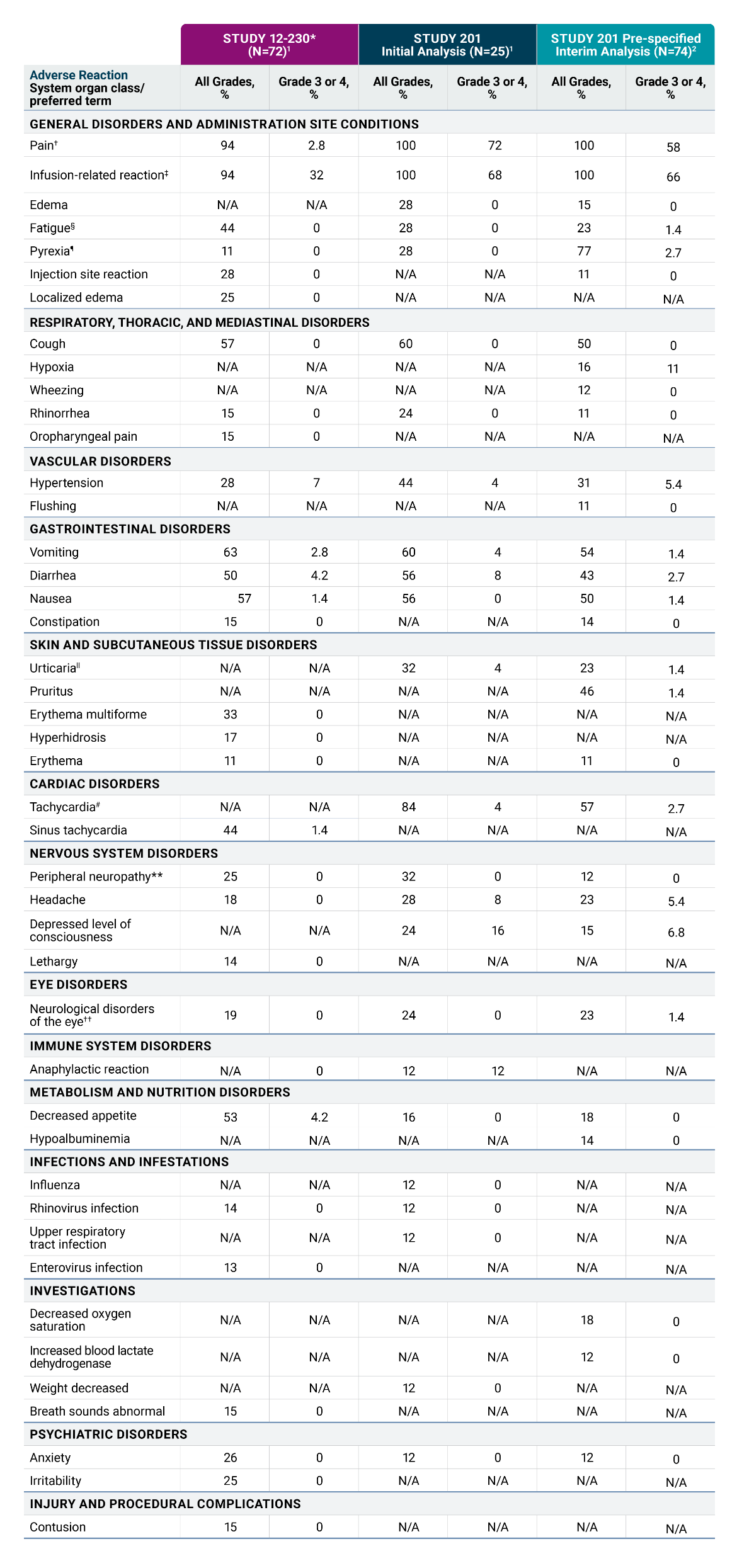
Serious infusion-related reactions occurred in 4% of patients in Study 201 and in 18% of patients in Study 12-230. Infusion-related reactions of any Grade occurred in 100% of patients in Study 201 and 94% of patients in Study 12-230. Hypotension of any grade occurred in 100% of patients in Study 201 and 89% of patients in Study 12-230.
In Study 201, 68% of patients experienced Grade 3 or 4 infusion reactions; and in Study 12-230, 32% of patients experienced Grade 3 or 4 infusion reactions. Anaphylaxis occurred in 12% of patients and two patients (8%) permanently discontinued DANYELZA due to anaphylaxis in Study 201. One patient in Study 12-230 (1.4%) experienced a Grade 4 cardiac arrest 1.5 hours following completion of DANYELZA infusion.
In Study 201, infusion reactions generally occurred within 24 hours of completing a DANYELZA infusion, most often within 30 minutes of initiation. Infusion reactions were most frequent during the first infusion of DANYELZA in each cycle. Eighty percent of patients required reduction in infusion rate and 80% of patients had an infusion interrupted for at least one infusion-related reaction.
Caution is advised in patients with pre-existing cardiac disease, as this may exacerbate the risk of severe hypotension.
Premedicate with an antihistamine, acetaminophen, an H2 antagonist and corticosteroid as recommended. Monitor patients closely for signs and symptoms of infusion reactions during and for at least 2 hours following completion of each DANYELZA infusion in a setting where cardiopulmonary resuscitation medication and equipment are available.
Reduce the rate, interrupt infusion, or permanently discontinue DANYELZA based on severity and institute appropriate medical management as needed.
Neurotoxicity
DANYELZA can cause severe neurotoxicity, including severe neuropathic pain, transverse myelitis, and reversible posterior leukoencephalopathy syndrome.
Pain
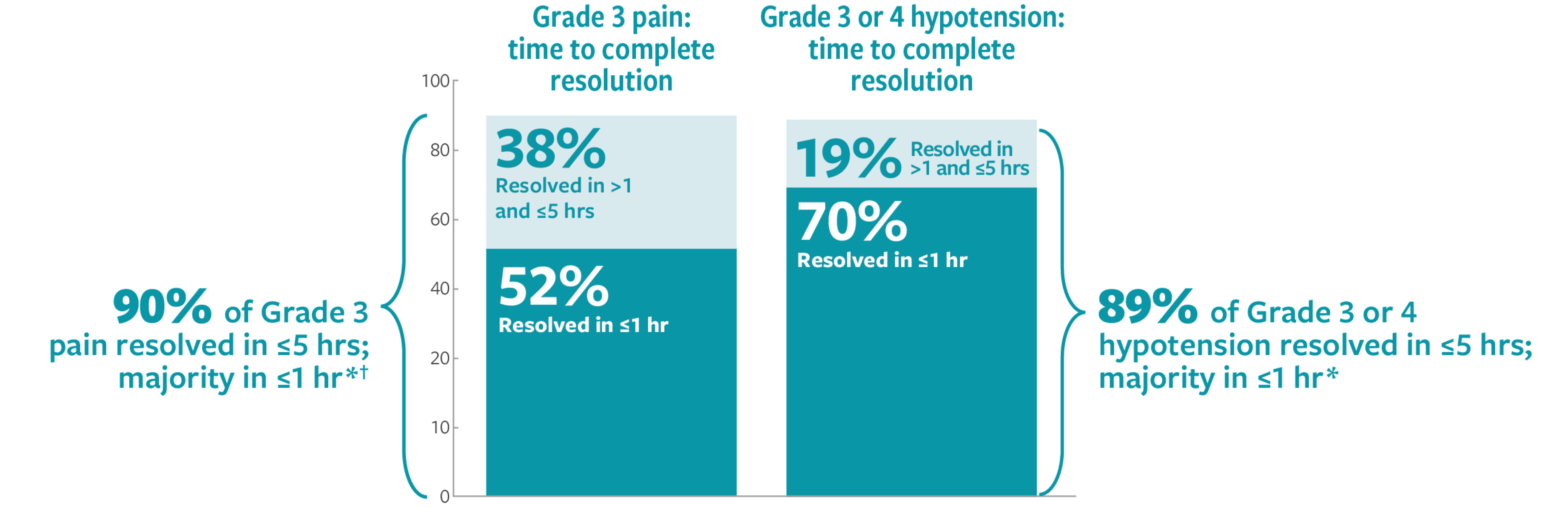
Pain, including abdominal pain, bone pain, neck pain, and extremity pain, occurred in 100% of patients in Study 201 and 94% of patients in Study 12-230. Grade 3 pain occurred in 72% of patients in Study 201. One patient in Study 201 (4%) required interruption of an infusion due to pain. Pain typically began during the infusion of DANYELZA and lasted a median of less than one day in Study 201 (range less than one day and up to 62 days).
Premedicate with drugs that treat neuropathic pain (e.g., gabapentin) and oral opioids. Administer intravenous opioids as needed for breakthrough pain. Permanently discontinue DANYELZA based on severity.
Transverse Myelitis
Transverse myelitis has occurred with DANYELZA. Permanently discontinue DANYELZA in patients who develop transverse myelitis.
Reversible Posterior Leukoencephalopathy Syndrome (RPLS)
Reversible posterior leukoencephalopathy syndrome (RPLS) (also known as posterior reversible encephalopathy syndrome or PRES) occurred in 2 (2.8%) patients in Study 12-230. Events occurred 2 and 7 days following completion of the first cycle of DANYELZA. Monitor blood pressure during and following DANYELZA infusion and assess for neurologic symptoms. Permanently discontinue DANYELZA in case of symptomatic RPLS.
Peripheral Neuropathy
Peripheral neuropathy, including peripheral sensory neuropathy, peripheral motor neuropathy, paresthesia, and neuralgia, occurred in 32% of patients in Study 201 and in 25% of patients in Study 12-230. Most signs and symptoms of neuropathy began on the day of the infusion and neuropathy lasted a median of 5.5 days (range 0 to 22 days) in Study 201 and 0 days (range 0 to 22 days) in Study 12-230.
Permanently discontinue DANYELZA based on severity.
Neurological Disorders of the Eye
Neurological disorders of the eye including unequal pupils, blurred vision, accommodation disorder, mydriasis, visual impairment, and photophobia occurred in 24% of patients in Study 201 and 19% of patients in Study 12-230. Neurological disorders of the eye lasted a median of 17 days (range 0 to 84 days) in Study 201 with two patients (8%) experiencing an event that had not resolved at the time of data cutoff, and a median of 1 day (range less than one day to 21 days) in Study 12-230. Permanently discontinue DANYELZA based on severity.
Prolonged Urinary Retention
Urinary retention occurred in 1 (4%) patient in Study 201 and in 3 patients (4%) in Study 12-230. All events in both studies occurred on the day of an infusion of DANYELZA and lasted between 0 and 24 days. Permanently discontinue DANYELZA in patients with urinary retention that does not resolve following discontinuation of opioids.
Myocarditis
Myocarditis has occurred in adolescent patients receiving DANYELZA in clinical trials and expanded access programs. Myocarditis occurred within days of receiving DANYELZA requiring drug interruption. Monitor for signs and symptoms of myocarditis during treatment with DANYELZA. Withhold, reduce the dose, or permanently discontinue DANYELZA based on severity.
Hypertension
Hypertension occurred in 44% of patients in Study 201 and 28% of patients in Study 12-230 who received DANYELZA. Grade 3 or 4 hypertension occurred in 4% of patients in Study 201 and 7% of patients in Study 12-230. Four patients (6%) in Study 12-230 permanently discontinued DANYELZA due to hypertension. In both studies, most events occurred on the day of DANYELZA infusion and occurred up to 9 days following an infusion of DANYELZA.
Do not initiate DANYELZA in patients with uncontrolled hypertension. Monitor blood pressure during infusion, and at least daily on Days 1 to 8 of each cycle of DANYELZA and evaluate for complications of hypertension including RPLS. Interrupt DANYELZA infusion and resume at a reduced rate, or permanently discontinue DANYELZA based on the severity.
Orthostatic Hypotension
Orthostatic hypotension has occurred in patients receiving DANYELZA in clinical trials and expanded access programs. Severe orthostatic hypotension, including cases requiring hospitalization, have occurred. Cases occurred within hours to 6 days of DANYELZA infusions in any cycle.
In patients with symptoms of orthostatic hypotension, monitor postural blood pressure prior to initiating treatment with DANYELZA and as clinically indicated with subsequent dosing. Withhold, reduce dose, or permanently discontinue DANYELZA based on severity.
Embryo-Fetal Toxicity
Based on its mechanism of action, DANYELZA may cause fetal harm when administered to a pregnant woman. Advise females of reproductive potential, including pregnant women, of the potential risk to a fetus. Advise females of reproductive potential to use effective contraceptive during treatment with DANYELZA and for two months after the last dose.
ADVERSE REACTIONS
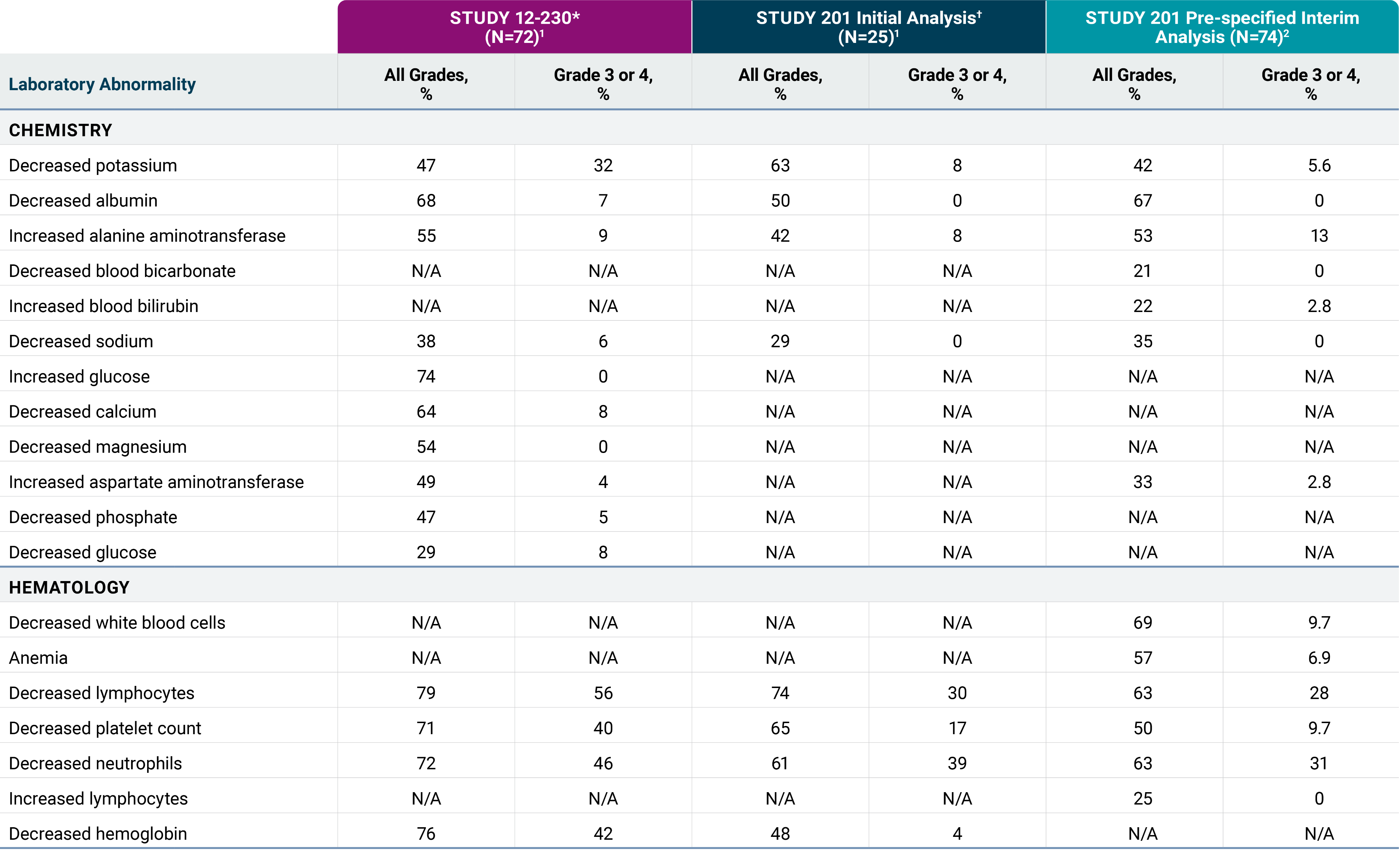
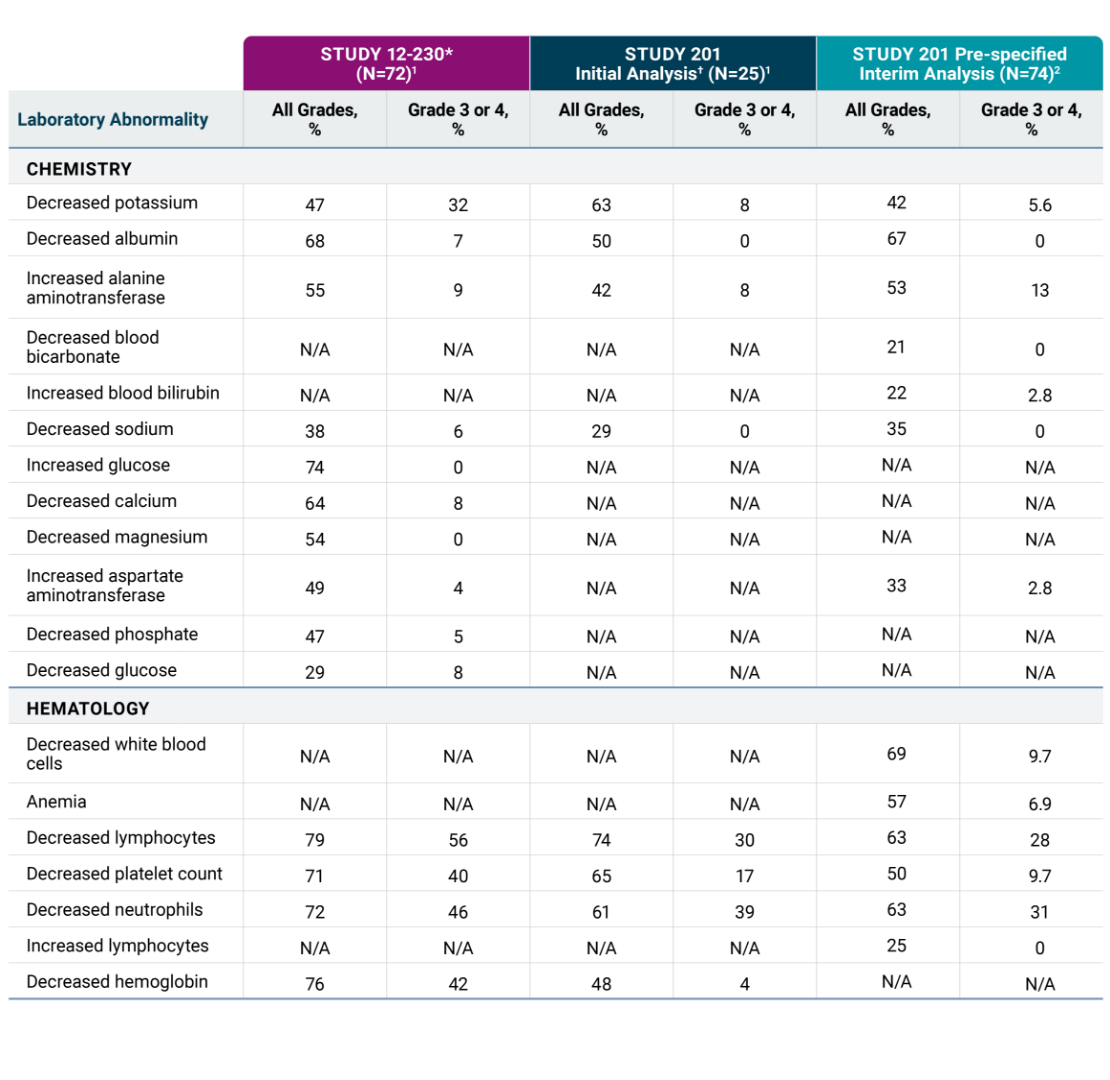
The most common adverse reactions in Studies 201 and 12-230 (≥25% in either study) were infusion-related reaction, pain, tachycardia, vomiting, cough, nausea, diarrhea, decreased appetite, hypertension, fatigue, erythema multiforme, peripheral neuropathy, urticaria, pyrexia, headache, injection site reaction, edema, anxiety, localized edema and irritability. The most common Grade 3 or 4 laboratory abnormalities (≥5% in either study) were decreased lymphocytes, decreased neutrophils, decreased hemoglobin, decreased platelet count, decreased potassium, increased alanine aminotransferase, decreased glucose, decreased calcium, decreased albumin, decreased sodium and decreased phosphate.
INDICATION
DANYELZA is indicated, in combination with granulocyte-macrophage colony-stimulating factor (GM-CSF), for the treatment of pediatric patients 1 year of age and older and adult patients with relapsed or refractory high-risk neuroblastoma in the bone or bone marrow who have demonstrated a partial response, minor response, or stable
disease to prior therapy.This indication is approved under accelerated approval based on overall response rate and duration of response. Continued approval for this indication may be contingent upon verification and description of clinical benefit in a confirmatory trial(s).
Please see full Prescribing Information and Patient Information for DANYELZA including Boxed Warning on serious infusion-related reactions and neurotoxicity.
References: 1. DANYELZA® [package insert]. New York, NY: Y-mAbs Therapeutics, Inc.; 2024.
2. Data on file. Y-mAbs Therapeutics, Inc.
References: 1. Data on file. Y-mAbs Therapeutics, Inc. 2. DANYELZA® [package insert]. New York, NY: Y-mAbs Therapeutics, Inc.; 2024. Available online at https://labeling.ymabs.com/danyelza. 3. Smith V, Foster J. High-risk neuroblastoma treatment review. Children (Basel). 2018;5(9):114. 4. Ahmed A, Zhang L, Reddivalla N, Hetherington M. Neuroblastoma in children: update on clinicopathologic and genetic prognostic factors. Pediatr Hematol Oncol. 2017;34(3):165-185. 5. London W, Castel V, Monclair T, et al. Clinical and biologic features predictive of survival after relapse of neuroblastoma: a report from International Neuroblastoma Risk Group project.
J Clin Oncol. 2011;29(24):3286-3292.
References: 1. DANYELZA® [package insert]. New York, NY: Y-mAbs Therapeutics, Inc.; 2024. Available online at https://labeling.ymabs.com/danyelza. 2. National Cancer Institute. Published November 27, 2017. Accessed May 17, 2021. Common Terminology Criteria for Adverse Events (CTCAE) Version 5.0. https://ctep.cancer.gov/protocolDevelopment/electronic_applications/docs/CTCAE_v5_Quick_Reference_8.5x11.pdf